Because humans and animals breathe and metabolize oxygen, they generate a variety of reactive oxygen species (ROS), or cell-damaging oxidants, as byproducts. Our bodies usually make enough antioxidants to counter that damage, but when that balance starts to favor oxidants, they can attack important biomolecules like proteins, nucleic acids, and lipids. That can lead to cancer, neurodegenerative disorders, and cardiovascular diseases.
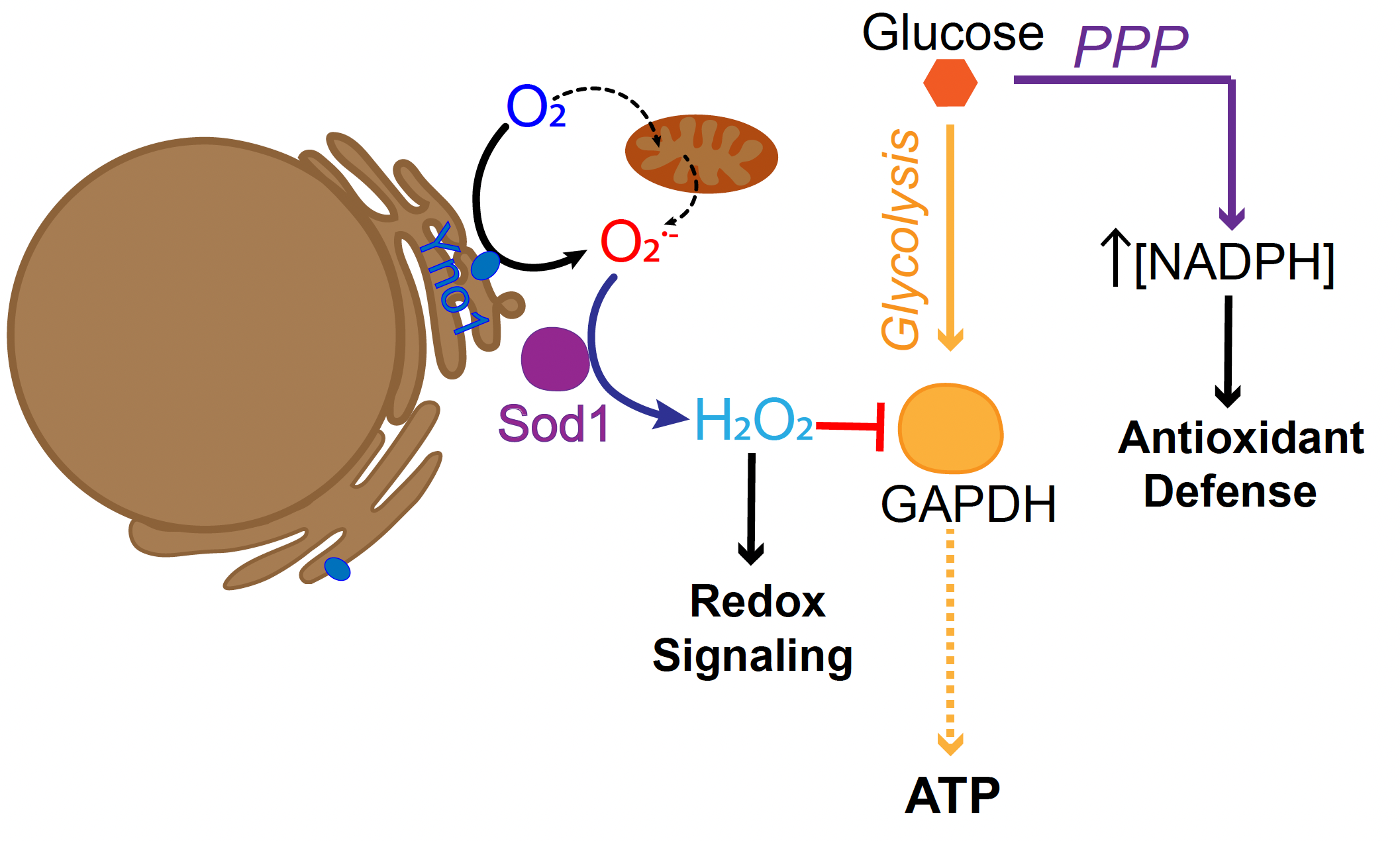
Fortunately, our bodies evolved to produce antioxidant enzymes such as Cu/Zn (copper/zinc) superoxide dismutase, or SOD1, which detoxifies certain harmful oxidants. In a weird twist, SOD1 is the only antioxidant enzyme that can take on one specific oxidant, superoxide, only to produce another ROS: hydrogen peroxide.
A team of Georgia Tech researchers have published a study that found an even stranger twist to this oxidant-antioxidant tale: SOD1 (good for cells) produces hydrogen peroxide (bad for cells) which stimulates the production of another important cellular antioxidant known as NADPH (also good for cells; more on this in a moment.)
“Yes, you heard that right,” says Amit Reddi, associate professor in the School of Chemistry and Biochemistry. “SOD1, an antioxidant enzyme, produces an oxidant, hydrogen peroxide, which in turn stimulates the production of another (good) antioxidant.”
Reddi is a co-author of this research along with Matthew Torres, associate professor in the School of Biological Sciences; Claudia Montllor-Albalate, former Reddi Lab member who received her Ph.D. in 2020 from the School of Chemistry and Biochemistry; Hyojung Kim, School of Chemistry and Biochemistry Ph.D. candidate; Annalise Thompson, a third-year graduate student in Reddi’s lab; and Alex Jonke, research scientist with the School of Biological Sciences.
Their study, “SOD1 Integrates Oxygen Availability to Redox Regulate NADPH Production and the Thiol Redoxome” is published in the Proceedings of the Natural Academy of Sciences (PNAS).
The NADPH/GAPDH connection
NADPH (nicotinamide adenine dinucleotide phosphate) is an important metabolite that is produced in cells. It provides a source of electrons that can act as an antioxidant and for the biosynthesis of numerous biomolecules, including fatty acids, amino acids, nucleotides, and cholesterol.
“NADPH is not only used as an antioxidant, but also to build new biomolecules to sustain cell proliferation,” Reddi says. “How do cells know to make enough NADPH to support aerobic life? We discovered that SOD1 senses oxygen availability via superoxide, and then converts this to hydrogen peroxide, which in turn inactivates an enzyme responsible for the breakdown of glucose, glyceraldehyde phosphate dehydrogenase (GAPDH).” That inactivation causes the build-up of metabolites that are re-routed to a pathway that synthesizes NADPH.
The story behind the SOD1 revelation
The PNAS research study began with a casual conversation in 2014 between Reddi and Torres at the former café in the Parker H. Petit Institute for Bioengineering and Biosciences (IBB).
“Given the very collaborative and collegial nature of faculty across the College of Sciences, and the Institute as a whole, it was easy to grab a coffee and discuss these ideas,” Reddi says. Work in the Reddi lab includes potential signaling roles for SOD1 and the hydrogen peroxide it produces; but understanding the extent to which these factors regulate signaling required a systems-level understanding of how widespread targets of SOD1 are in a cell.
Torres focuses on mass spectrometry-based proteomics (the study of all proteins produced and modified by an organism or system) to probe cell-wide signaling networks, so it seemed to Reddi like a perfect fit.
Then, Reddi says, Montllor-Albalate made the discovery that SOD1-derived hydrogen peroxide can regulate NADPH production and adaptation to aerobic life. Meanwhile, Kim, a joint student of the Reddi and Torres labs, drove the work to identify proteome-wide targets of SOD1-derived hydrogen peroxide.
The conversation in IBB led to a 2016 grant from the National Institutes of Health to study the topic further. The resulting paper “we feel will make a strong impact in the field of redox biology and signaling,” Reddi adds.
SOD1’s potential in future cancer therapy
SOD1 is often thought of as an appealing anti-cancer therapeutic because of its ability to scavenge superoxides. The theory is that if SOD1 is inactivated, cancer cells will be at a disadvantage.
Reddi says his team’s results “suggest this very simple approach may need to be reconsidered, because the hydrogen peroxide that is produced by SOD1 plays broader roles in metabolism — and regulates many other enzymes and pathways. For instance, many cancer cells are addicted to glucose (sugars) and have an increased reliance on it for energy and metabolism, with GAPDH being a key enzyme in the process. Our findings that SOD1-derived hydrogen peroxide inactivates GAPDH would suggest that inhibiting SOD1 in certain cancers could actually result in elevated GAPDH activity, and increased metabolism of glucose, which may be detrimental in fighting cancer.”
Torres and Reddi are continuing their collaboration to investigate other aspects of SOD1 and hydrogen peroxide signaling in cancer metabolism and its implications for disease progression.
doi.org/10.1073/pnas.2023328119
This work was supported by GM118744 to Reddi and Torres, and Blanchard Fellowship to Reddi.
For More Information Contact
Renay San Miguel
Communications Officer II/Science Writer
College of Sciences
404-894-5209